
The fascinating science of UV absorption by electrons and the principles of quantum based photo-protection of plants.
Sun is the single biggest source of energy on our planet. It is the energy that is used to power the growth of the greatest, by mass, kingdom on Earth – plants.
As stationary organisms, depending on sunlight for survival, plants are in a constant state of balancing between harvesting light in order to grow and overcoming the hazards caused by excess radiation.
Plants basking in the sun are constantly bombarded by photons of light, some of which are used to power up the photosynthesis, but others pose a grave danger.
Unfiltered UV rays give rise to free radicals that can wreak havoc inside the plants cells, damaging its sensitive machinery, DNA and eventually causing the plants death…
UV Absorption: Quantum Physics and the laws of Electron Transitions.
Unable to run and hide, plants evolved some of the most highly sophisticated mechanisms that allows them to not only survive in excessive light conditions, but even thrive. They do that by cleverly producing their own sunscreen – a class of molecules with so called pi-electron systems. These molecules absorb harmful wavelengths by employing the laws of quantum mechanics and electron excitation.
When the UV light hits molecules with pi-electron systems, it causes their electrons to change position, thereby absorbing harmful UV energy and dissipating it as heat.
However ingenious, this is only one part of the plant’s strategy. To combat UV caused stress, plants also produce a diverse range of antioxidants. These molecules disarm free radicals as well as help limit, repair and even reverse sun induced damage.
QUANTUM THEORY OF LIGHT
According to the Quantum Theory of Light, electromagnetic radiation has characteristics of both particles and waves, and energy is transferred only in certain well defined quantities.
BOHR MODEL OF ATOM
In the Bohr model of Atom, electrons can travel in one of 4 orbitals around the nucleus, where each orbital is associated with a specific energy level, dictated by the unique composition of the nucleus.
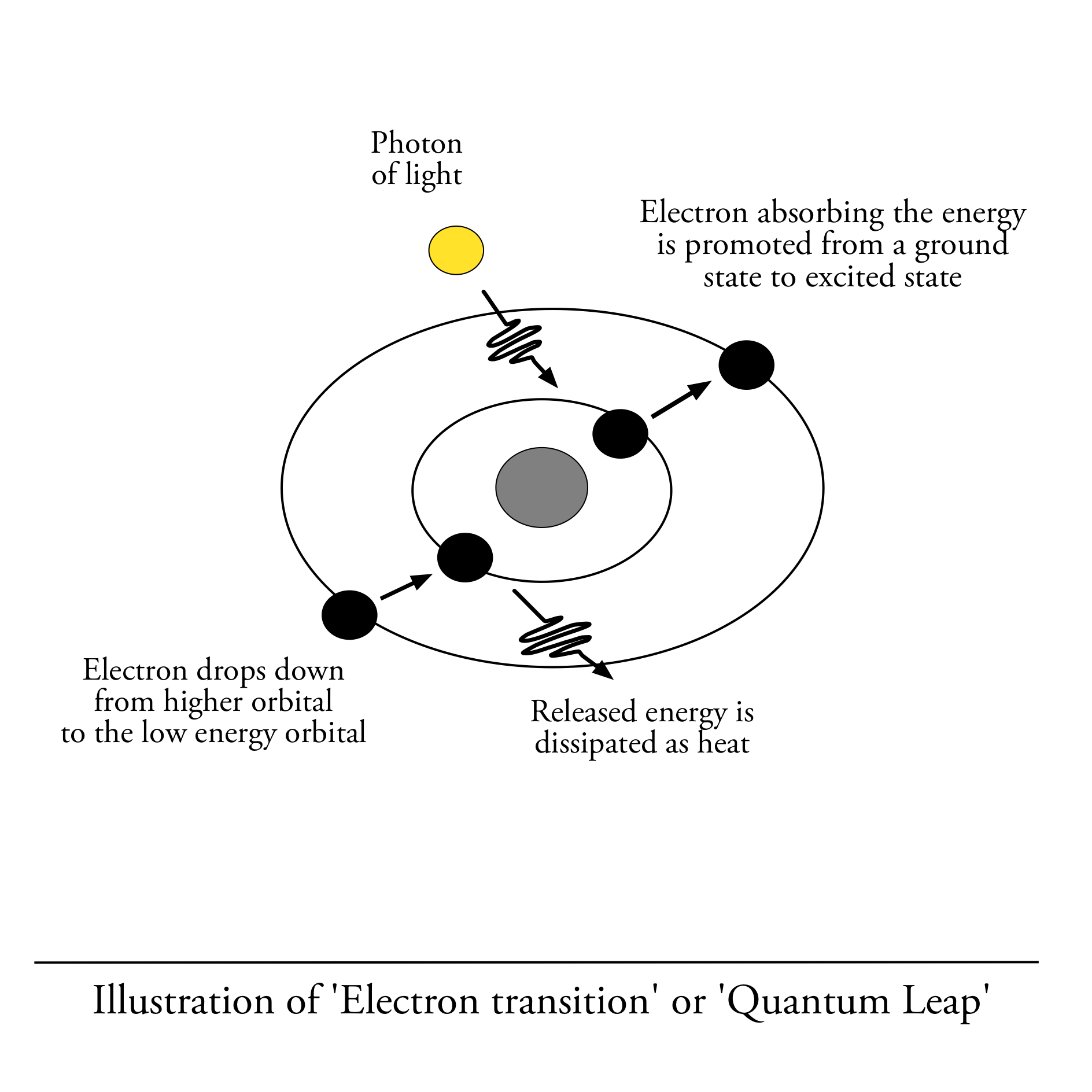
When an electron absorbs a photon of light that equals the energy difference between orbitals, the absorbed energy causes it to become excited and jump to a higher energy orbit in what is called a “quantum leap”. Absorption of energy by the electron is what produces the sunscreening effect and what we measure by spectroscopy.
ELECTRON TRANSITION
A class of molecules that absorb light in the Ultra Violet and Visible Light spectrum are compounds with Pi-electron systems, as the energy needed to excite Pi-electrons corresponds to the energy of photons in the UV spectrum.
When electron in Pi-electron systems absorbs a photon of light, it jumps into a higher energy orbital in a move called Quantum Leap
